Archive
Lexaria Reports Potentially Ground-Breaking Findings in Sildenafil Animal Study
 | |||||||||
 |  | ||||||||
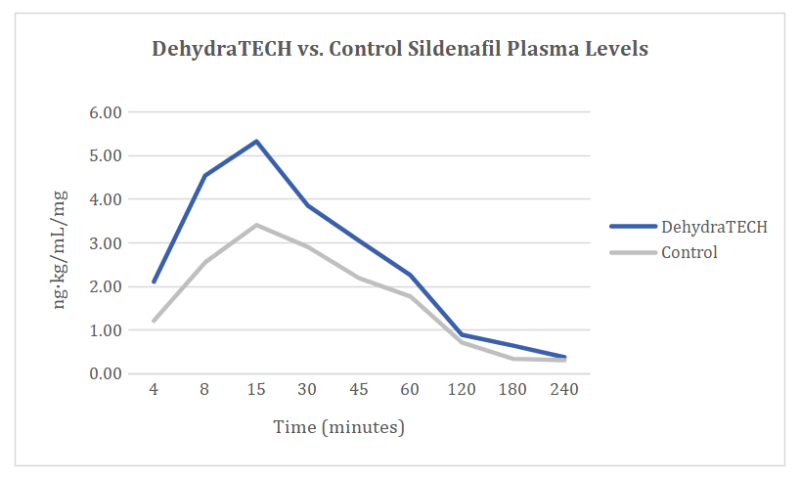
DehydraTECHTM-sildenafil delivered 74% more drug at 4 minutes, than the control
Kelowna, British Columbia - TheNewswire – February 2, 2022 – Lexaria Bioscience Corp. (Nasdaq: LEXX) (Nasdaq: LEXXW) (the “Company” or “Lexaria”), a global innovator in drug delivery platforms announces positive findings in an animal study evaluating DehydraTECHTM processing of the phosphodiesterase inhibitor (“PDE5 inhibitor”) sildenafil, for potential application in the management of erectile dysfunction.
A clear trend toward faster and higher overall delivery of sildenafil into the bloodstream was evidenced over the course of the study. In as little as four minutes after dosing, the DehydraTECH formulation delivered 74% more sildenafil into the bloodstream on average than the concentration-matched, generic control formulation. Seven minutes after dosing, the DehydraTECH-sildenafil formulation achieved an average blood level higher than the generic sildenafil control formulation reached at any point during the study.
The DehydraTECH-sildenafil formulation achieved a maximum concentration in the bloodstream (or “Cmax”) that was roughly 70% higher than that achieved with the generic sildenafil control formulation (i.e., DehydraTECH-sildenafil Cmax = 5.93 ± 6.53 ng·kg/mL/mg versus Control-sildenafil Cmax = 3.48 ± 1,83 ng·kg/mL/mg).
The time at which the Cmax was reached on average across all of the animals in the study (“Tmax”) was roughly 25% faster at 15.1 ± 5.9 minutes with the DehydraTECH-sildenafil versus 21 ± 7.74 minutes with the Control-sildenafil. Also, the total sildenafil delivery over time or the Area Under the Curve (“AUC”) was higher with roughly a 37% improvement evident over the control formulation (i.e., DehydraTECH-sildenafil AUC = 6.35 ± 3.04 hr·kg·ng/mL/mg versus Control-sildenafil AUC = 4.62 ± 2.04 hr·kg·ng/mL/mg).
The most well-known sildenafil product for sale in the world today is Viagra®. Sildenafil is moderately bioavailable orally at roughly 40%, but many people find it slow to act. Lexaria’s study findings could pave the way for development of faster and better acting sildenafil oral formulations, whether for the Viagra® branded product or its generic pharmaceutical competitors.
Study PDE5-A21-1 was an animal study conducted at a US-based, third-party independent laboratory, in which twenty male Sprague-Dawley rats (two groups of 10 rats each) were treated with a single dose of the DehydraTECH-sildenafil and Control-sildenafil formulations described herein. Of note, the reported improvements in delivery rate including the Cmax, Tmax and AUC evidenced in the study did not achieve statistical significance, therefore, supporting further investigation in a larger number of animals.
Lexaria will provide additional information of further developments or plans to pursue expanded investigation with DehydraTECH for PDE5 inhibitors as they materialize.
About Lexaria Bioscience Corp.
Lexaria Bioscience Corp.’s patented drug delivery technology, DehydraTECH™, improves the way active pharmaceutical ingredients (APIs) enter the bloodstream by promoting more effective oral delivery. Since 2016, DehydraTECH has repeatedly demonstrated the ability to increase bio-absorption with cannabinoids and nicotine by 5-10x and, in some instances with cannabinoids by as much as 27x compared to standard industry formulations, reduce time of onset from 1 - 2 hours to minutes, and mask unwanted tastes; and is also being evaluated for orally administered antiviral drugs, non-steroidal anti-inflammatory drugs (NSAIDs), PDE5 inhibitors and more. DehydraTECH has also evidenced an ability to deliver some drugs more effectively across the blood brain barrier. Lexaria operates a licensed in-house research laboratory and holds a robust intellectual property portfolio with 23 patents granted and over 50 patents pending worldwide. For more information, please visit www.lexariabioscience.com.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This press release includes forward-looking statements. Statements as such term is defined under applicable securities laws. These statements may be identified by words such as "anticipate," "if," "believe," "plan," "estimate," "expect," "intend," "may," "could," "should," "will," and other similar expressions. Such forward-looking statements in this press release include, but are not limited to, statements by the company relating the Company’s ability to carry out research initiatives, receive regulatory approvals or grants or experience positive effects or results from any research or study. Such forward-looking statements are estimates reflecting the Company's best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that the Company will actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements. As such, you should not place undue reliance on these forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation and regulatory approvals, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval process, potential adverse effects arising from the testing or use of products utilizing the DehydraTECH technology, the Company’s ability to maintain existing collaborations and realize the benefits thereof, delays or cancellations of planned R&D that could occur related to pandemics or for other reasons, and other factors which may be identified from time to time in the Company's public announcements and periodic filings with the US Securities and Exchange Commission on EDGAR. There is no assurance that any of Lexaria’s postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease. Any forward-looking statements contained in this release speak only as of the date hereof, and the Company expressly disclaims any obligation to update any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise, except as otherwise required by law.
George Jurcic - Head of Investor Relations