Archive
Lexaria's Hormone Study Shows Significant Enhancement in Oral Estradiol Delivery
 | |||||||||
 |  | ||||||||
900% and 2,000% improvements in peak bloodstream delivery using DehydraTECH
Kelowna, BC - TheNewswire - May 18, 2023 - Lexaria Bioscience Corp. (Nasdaq:LEXX) (Nasdaq:LEXXW) (the “Company” or “Lexaria”), a global innovator in drug delivery platforms announces that its animal study HOR-A22-1 has successfully completed, showing significant enhancement in the oral delivery of the estrogen hormone estradiol, an important component of therapeutic products in the women’s health sector.
The DehydraTECH-estradiol formulation achieved an average peak concentration in the bloodstream (or "Cmax") of 5.65ng/mL that was roughly nine times (900%) higher than that achieved with the control formulation at only 0.63 ng/mL. As well, because estradiol is known to be quickly converted into the metabolite estrone by cells in the uterus, mammary glands and liver, estrone levels were also quantified in the study. This revealed that levels of the estrone metabolite were also significantly higher comparing an average Cmax of 6.49 ng/mL with the DehydraTECH formulation to only 0.302 ng/mL achieved with the control, representing greater than a twenty-fold (2,000%) improvement in delivery.
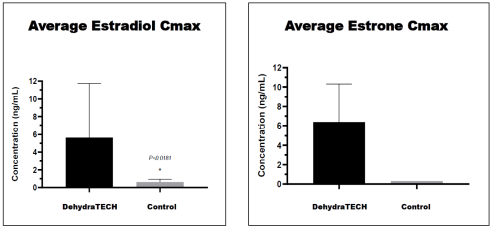
Average Estradiol and Estrone Cmax in Blood Plasma Following DehydraTECH-Estradiol and Control Administration
Also, the total estradiol and estrone recovery in the blood plasma over time or the Area Under the Curve ("AUC") was dramatically higher at 3.9 hr·ng/mL for estradiol and 32.6hr·ng/mL for the estrone with the DehydraTECH composition versus being non-detectable below the lower limit of quantitation of the assay (i.e., 0.25 ng/mL) with the control in both instances. This means that the AUC findings were at least fifteen times (1,500%) greater than the control for estradiol and over one hundred and twenty-five times (12,500%) greater for estrone.
Oral estradiol therapy is commonly used by women in birth control products, as well as to help reduce the symptoms of menopause and protect bone health. The hormone replacement market is estimated at $46.5 billion in 2027.
Oral estradiol formulations are known to have very low bioavailability, generally in the 5% range, necessitating relatively high dosages to achieve the desired beneficial effects. This, in turn, can lead to unwanted side effects ranging from gastrointestinal complications such as nausea, vomiting, diarrhea, stomach cramps and bloating, to other issues such as headache, dizziness, rash and more. Lexaria’s DehydraTECH processing may offer a new approach to formulating estradiol and potentially other human hormone therapies for enhanced oral delivery toward improved safety and efficacy if pursued and proven ultimately clinically in humans.
According to a study just published in the Canadian Medical Association Journal, “menopause and perimenopause can be associated with distressing symptoms and reduced quality of life”. As reported by CTV News, “more women suffering from debilitating symptoms of menopause should be presented with the option of hormonal therapy”.
About Estrogen
There are three main types of Estrogen: They are Estrone (E1) which is the only estrogen that females continue to make after menopause and which males and females manufacture in the adrenal glands; Estradiol (E2) which is the most common form of estrogen in women of childbearing age and is primarily made in the ovaries, and in men, is manufactured in very small quantities in the testicles; and Estriol (E3) that is primarily made in the placenta of pregnant women.
About the Study
Study HOR-A22-1 was a pharmacokinetic (“PK”) study performed in twenty female Sprague-Dawley rats in order to evaluate the ability of DehydraTECH™ to enhance the delivery characteristics of orally administered estradiol. Delivery was assessed comparing a DehydraTECH-estradiol composition to a concentration-matched generic estradiol preparation lacking the DehydraTECH formulation and processing enhancements. Formulations were administered orally at a dose of 10 mg/kg of estradiol. Following dosing, blood was collected up to 48 hours post-dose. The concentration of estradiol and estrone were then determined in plasma following dosing using a validated liquid chromatography tandem mass spectrometry “LC-MS/MS”) method.
Study HOR-A22-1 was conducted at a U.S.-based, third-party independent laboratory. Lexaria will provide additional information of further developments or plans to pursue expanded investigation with DehydraTECH for human hormone therapies as they become available.
About DehydraTECH
DehydraTECH is a patented drug delivery formulation and processing platform technology Lexaria has developed and is investigating for a variety of beneficial molecules. DehydraTECH is designed to improve the way active molecules enter the bloodstream upon oral ingestion. DehydraTECH has also demonstrated enhanced delivery of certain active molecules into brain tissue, which Lexaria believes to be of particular importance for centrally active compounds. Lexaria has also developed DehydraTECH formulations for other applications demonstrating superior bio-absorption when administered intraorally and topically.
About Lexaria Bioscience Corp.
Lexaria Bioscience Corp.’s patented drug delivery technology, DehydraTECH™, improves the way active pharmaceutical ingredients (APIs) enter the bloodstream through oral delivery. Since 2016, DehydraTECH has repeatedly demonstrated the ability to increase bio-absorption with cannabinoids, antiviral drugs, PDE5 inhibitors and more. DehydraTECH has also evidenced an ability to deliver some drugs more effectively across the blood brain barrier. Lexaria operates a licensed in-house research laboratory and holds a robust intellectual property portfolio with 30 patents granted and many patents pending worldwide. For more information, please visit www.lexariabioscience.com.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This press release includes forward-looking statements. Statements as such term is defined under applicable securities laws. These statements may be identified by words such as "anticipate," "if," "believe," "plan," "estimate," "expect," "intend," "may," "could," "should," "will," and other similar expressions. Such forward-looking statements in this press release include, but are not limited to, statements by the company relating the Company’s ability to carry out research initiatives, receive regulatory approvals or grants or experience positive effects or results from any research or study. Such forward-looking statements are estimates reflecting the Company's best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that the Company will actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements. As such, you should not place undue reliance on these forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation and regulatory approvals, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval process, potential adverse effects arising from the testing or use of products utilizing the DehydraTECH technology, the Company’s ability to maintain existing collaborations and realize the benefits thereof, delays or cancellations of planned R&D that could occur related to pandemics or for other reasons, and other factors which may be identified from time to time in the Company's public announcements and periodic filings with the US Securities and Exchange Commission on EDGAR. The Company provides links to third-party websites only as a courtesy to readers and disclaims any responsibility for the thoroughness, accuracy or timeliness of information at third-party websites. There is no assurance that any of Lexaria’s postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease. Any forward-looking statements contained in this release speak only as of the date hereof, and the Company expressly disclaims any obligation to update any forward-looking statements or links to third-party websites contained herein, whether as a result of any new information, future events, changed circumstances or otherwise, except as otherwise required by law.
INVESTOR CONTACT: